Barostim Therapy for Heart Failure: What You Need to Know
We recently had a question about Barostim from a community member. I will be honest – I had never heard that term before. So I decided to do some research. I learned that this is a new device that is now an option for some people with heart failure! Here is what I learned.
What is Barostim?
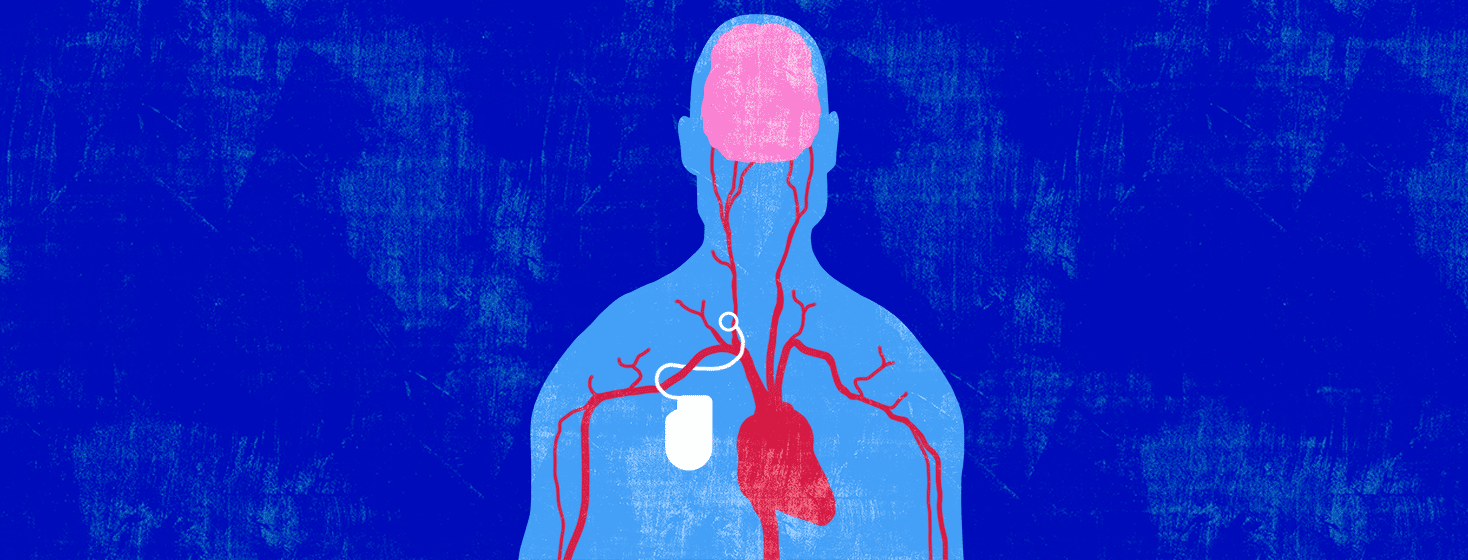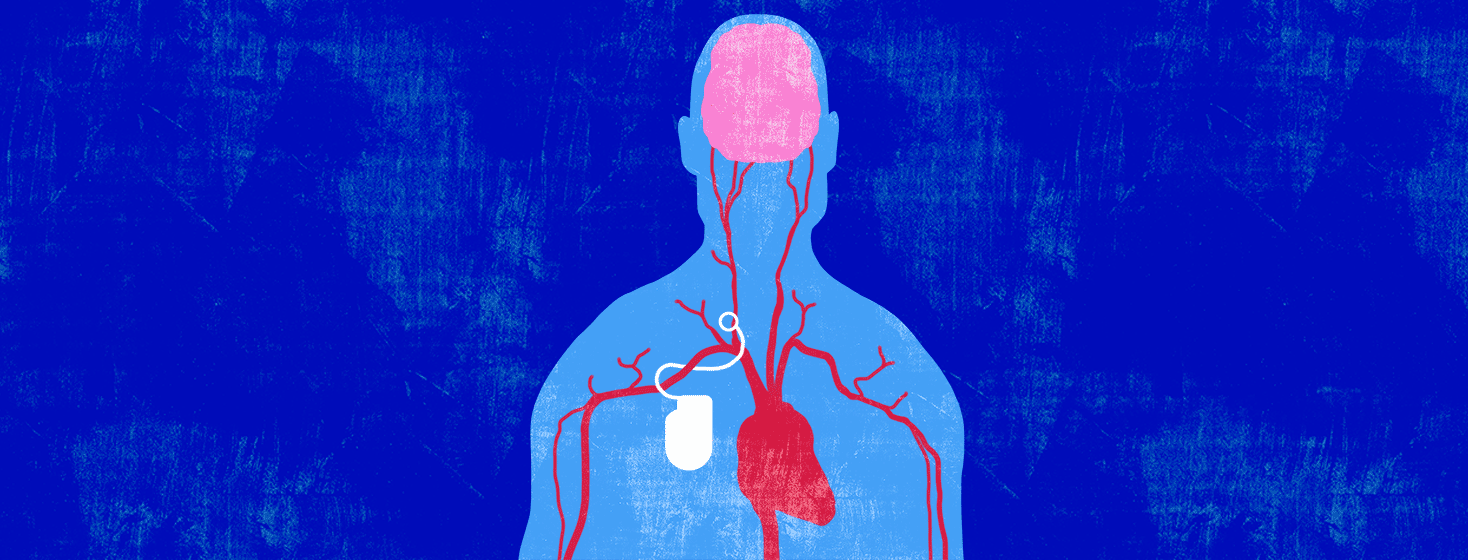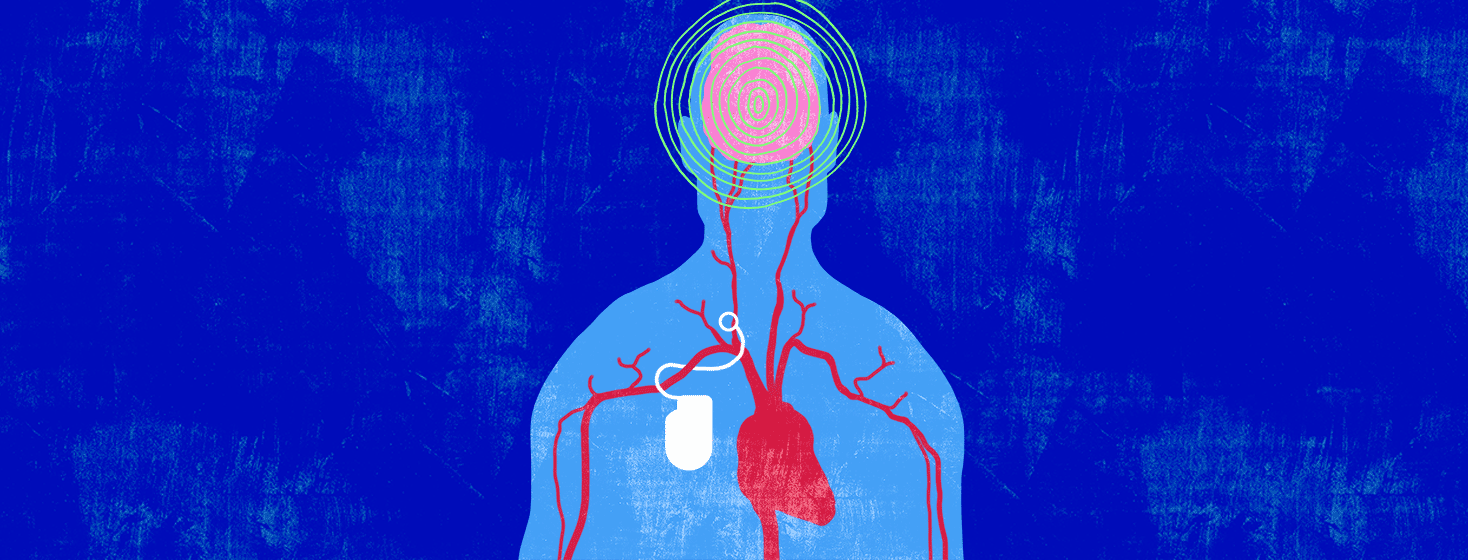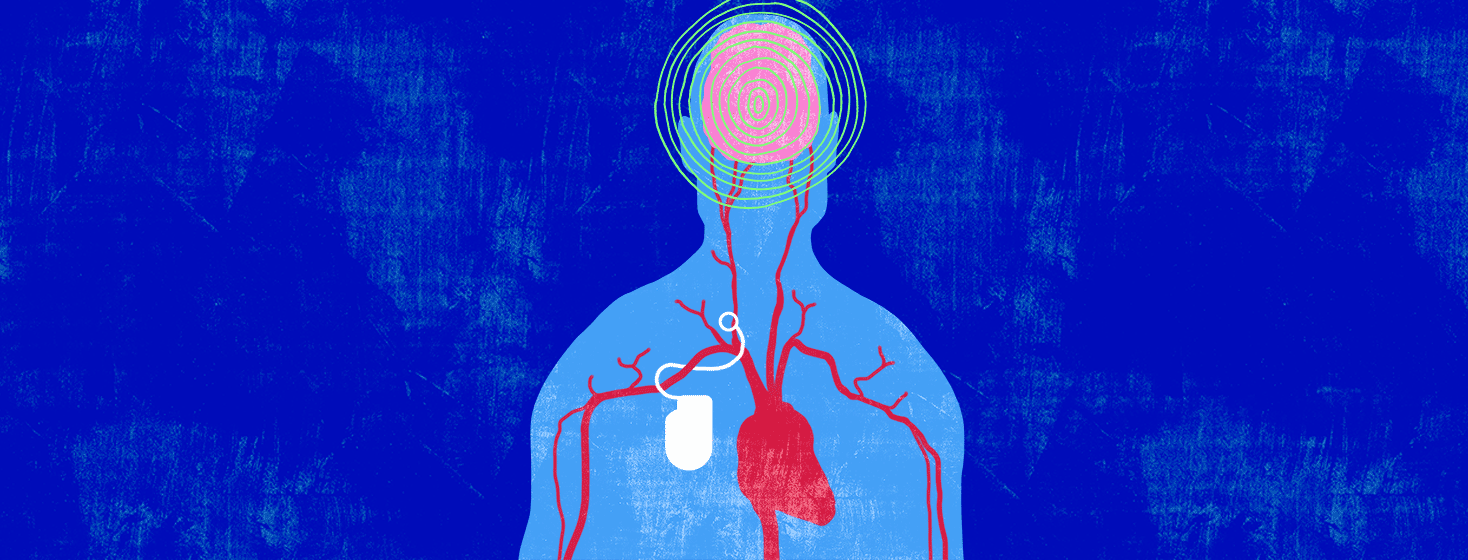
The procedure is called Barostim Activation Therapy. The device used is the Barostim Neo System. This device was approved by the US Food and Drug Administration (FDA) in 2019. It is an implantable device, inserted inside your chest just under your right collarbone. It is connected by thin wires to your carotid artery.1-3
Arteries are blood vessels that carry freshly oxygenated blood through your body. Lining your arteries are baroreceptors. These are receptors that monitor your blood pressure. When your blood pressure gets too high, your baroreceptors send a signal to a specialized region of your brain.1,4
In response, this specialized region of your brain sends signals to various organs of your body, causing your blood vessels to dilate. This means that your blood vessels are told to relax. When your blood vessels are relaxed, your heart doesn’t have to work as hard to pump blood through them.1,4
Some heart diseases cause this baroreceptor system to not work as well as it should. This may cause a person to develop high blood pressure (hypertension) that is resistant to treatment. It may also cause a worsening of heart failure symptoms, such as fatigue and shortness of breath.1
This or That
Have you heard of Barostim Therapy?
What does the device do?
The device monitors your blood pressure. When your blood pressure is high, an electrical impulse is sent to your carotid baroreceptors. This:1
- Causes your blood vessels to dilate, which
- Lowers your blood pressure and heart rate, which
- Helps your heart to relax
The device is used along with medicinal treatment, such as diuretics, ACE inhibitors, and beta blockers. Which medicines are best for you will be determined by your individual cardiologist. Studies show that the device is effective at lowering blood pressure when used along with medicine. It is also effective at improving symptoms of heart failure. For example, people with heart failure were able to walk farther without experiencing as much shortness of breath and fatigue. Studies also showed that it was effective at improving overall quality of life for those living with heart failure.1,3
The FDA approved the device in 2019 through the Breakthrough Device Designation. This means that early studies showed that the device was safe and worked so well that the FDA wanted to make it available right away as an option for those who qualify.3,5
Who qualifies for Barostim?
Barostim is not for all patients with heart failure. At the present time, it is only recommended for patients who:3,6-10
- Have a diagnosis of advanced heart failure. This means you experience symptoms of heart failure despite treatment with medicine. These symptoms limit your ability to stay physically active and decrease your overall quality of life.
- Have a diagnosis of heart failure with reduced ejection fraction (HFREF). The ejection fraction (EF) of your left ventricle must be 35 percent or less. EF is a measurement of how much blood your heart pumps out with each heartbeat. Normal is 55 to 75 percent.
- Do not qualify for resynchronization therapy (or resynchronization therapy was attempted and did not work). This therapy is treatment for HFREF with pacemakers or implantable cardioversion devices.
This is good news!
The FDA was very impressed by early study results using the Barostim Neo. So, rather than waiting for further testing, they have made it available as an option for you to try if you meet the above criteria. But the FDA is also requiring the manufacturer to continue conducting studies on this device to further prove that it both prolongs life and reduces hospitalizations. As these studies conclude, and if they continue to show impressive results, the FDA may be forced to increase the availability of this device.
This or That
Will you be talking to your doctor about Barostim Therapy?
Do you have a heart failure story? Click the button below to share with our community!
Join the conversation